DMT-2'-O-TBDMS-C(Ac)-CE-Phosphoramidite
DMT-2'-O-TBDMS-C(Ac)-CE-Phosphoramidite - N (Normal) / 0.25g / 30mL screwed bottle-28 is backordered and will ship as soon as it is back in stock.
Couldn't load pickup availability
Shipping notes
Shipping notes
Related Products
Details
Details
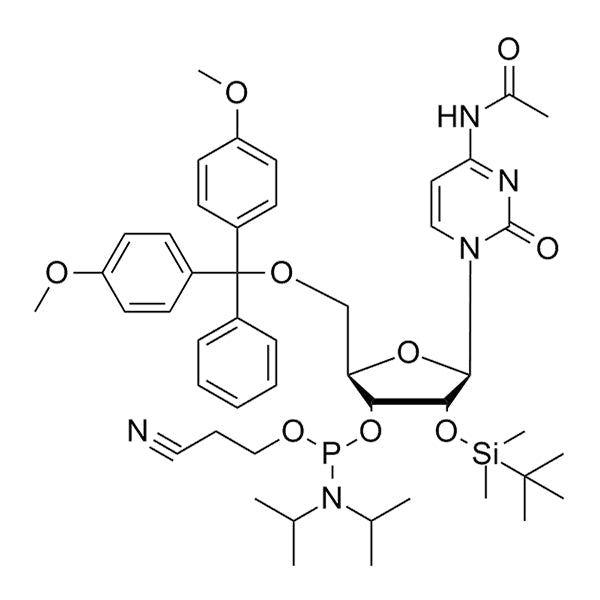
DMT-2'-O-TBDMS-C(Ac)-CE-Phosphoramidite is a protected ribonucleoside building block used for solid-phase RNA oligonucleotide synthesis. It contains a 5'-dimethoxytrityl (DMT) protecting group for 5'-hydroxyl protection, a 2'-O-tert-butyldimethylsilyl (TBDMS) protecting group for 2'-hydroxyl protection, a cyanoethyl (CE) protecting group on phosphite, and a acetyl (Ac) base-protecting group on cytosine.
TBDMS offers excellent stability for protecting 2'-hydroxyl of ribonucleosides during acid detritylation and oxidation steps.
TBDMS deprotection can be achieved using fluoride reagents (e.g., tetrabutylammonium fluoride (TBAF) and triethylamine trihydrofluoride (TEA·3HF)), which can cleave the silyl group without damaging the RNA backbone.
Applications
Synthetic RNA oligonucleotides
Monomer for preparing functional RNA sequences used in biochemical assays and binding studies,[1] such as RNA aptamers and ribozymes.
Mixed backbone design
Enables the synthesis of RNA-DNA chimeras, e.g., chimeric RNA/DNA oligonucleotide-based gene therapy.[2][3]
Features and Benefits
Other Notes
- Storage: Store in a dry, inert atmosphere at -20 °C.
- Coupling: 12 minutes coupling time recommended
- Compatibility: Can be used alongside modified phosphoramidites (e.g., 2′-OMe, 2′-MOE, Locked-NA) to synthesize chimeric oligonucleotides.
Reference
Specifications
Specifications
-
Catalog No.PR3-008PR3-008PR3-008PR3-008PR3-008PR3-008PR3-008PR3-008
-
CAS No.121058-88-6121058-88-6121058-88-6121058-88-6121058-88-6121058-88-6121058-88-6121058-88-6
-
SMILESC[Si](C)(C(C)(C)C)O[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=OC[Si](C)(C(C)(C)C)O[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=OC[Si](C)(C(C)(C)C)O[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=OC[Si](C)(C(C)(C)C)O[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=OC[Si](C)(C(C)(C)C)O[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=OC[Si](C)(C(C)(C)C)O[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=OC[Si](C)(C(C)(C)C)O[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=OC[Si](C)(C(C)(C)C)O[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=O
-
Molecular FormulaC47H64N5O9PSiC47H64N5O9PSiC47H64N5O9PSiC47H64N5O9PSiC47H64N5O9PSiC47H64N5O9PSiC47H64N5O9PSiC47H64N5O9PSi
-
Molecular Weight902.1902.1902.1902.1902.1902.1902.1902.1
-
AppearanceWhite to faint yellow powderWhite to faint yellow powderWhite to faint yellow powderWhite to faint yellow powderWhite to faint yellow powderWhite to faint yellow powderWhite to faint yellow powderWhite to faint yellow powder
-
PurityHPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%
-
Storage Condition-20℃-20℃-20℃-20℃-20℃-20℃-20℃-20℃
-
Moisture ContentK.F.≤0.20% w/wK.F.≤0.20% w/wK.F.≤0.20% w/wK.F.≤0.20% w/wK.F.≤0.20% w/wK.F.≤0.20% w/wK.F.≤0.20% w/wK.F.≤0.20% w/w
Documentation
Documentation
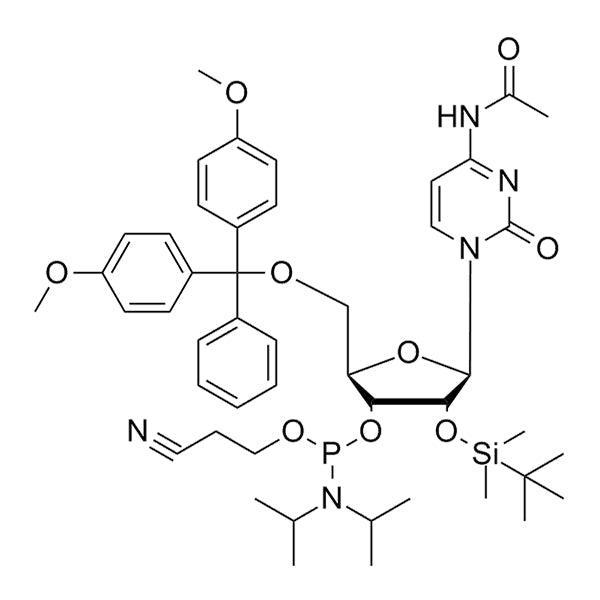
DMT-2'-O-TBDMS-C(Ac)-CE-Phosphoramidite is a protected ribonucleoside building block used for solid-phase RNA oligonucleotide synthesis. It contains a 5'-dimethoxytrityl (DMT) protecting group for 5'-hydroxyl protection, a 2'-O-tert-butyldimethylsilyl (TBDMS) protecting group for 2'-hydroxyl protection, a cyanoethyl (CE) protecting group on phosphite, and a acetyl (Ac) base-protecting group on cytosine.
TBDMS offers excellent stability for protecting 2'-hydroxyl of ribonucleosides during acid detritylation and oxidation steps.
TBDMS deprotection can be achieved using fluoride reagents (e.g., tetrabutylammonium fluoride (TBAF) and triethylamine trihydrofluoride (TEA·3HF)), which can cleave the silyl group without damaging the RNA backbone.
Applications
Synthetic RNA oligonucleotides
Monomer for preparing functional RNA sequences used in biochemical assays and binding studies,[1] such as RNA aptamers and ribozymes.
Mixed backbone design
Enables the synthesis of RNA-DNA chimeras, e.g., chimeric RNA/DNA oligonucleotide-based gene therapy.[2][3]
Features and Benefits
Other Notes
- Storage: Store in a dry, inert atmosphere at -20 °C.
- Coupling: 12 minutes coupling time recommended
- Compatibility: Can be used alongside modified phosphoramidites (e.g., 2′-OMe, 2′-MOE, Locked-NA) to synthesize chimeric oligonucleotides.
Reference
-
Catalog No.PR3-008PR3-008PR3-008PR3-008PR3-008PR3-008PR3-008PR3-008
-
CAS No.121058-88-6121058-88-6121058-88-6121058-88-6121058-88-6121058-88-6121058-88-6121058-88-6
-
SMILESC[Si](C)(C(C)(C)C)O[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=OC[Si](C)(C(C)(C)C)O[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=OC[Si](C)(C(C)(C)C)O[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=OC[Si](C)(C(C)(C)C)O[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=OC[Si](C)(C(C)(C)C)O[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=OC[Si](C)(C(C)(C)C)O[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=OC[Si](C)(C(C)(C)C)O[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=OC[Si](C)(C(C)(C)C)O[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=O
-
Molecular FormulaC47H64N5O9PSiC47H64N5O9PSiC47H64N5O9PSiC47H64N5O9PSiC47H64N5O9PSiC47H64N5O9PSiC47H64N5O9PSiC47H64N5O9PSi
-
Molecular Weight902.1902.1902.1902.1902.1902.1902.1902.1
-
AppearanceWhite to faint yellow powderWhite to faint yellow powderWhite to faint yellow powderWhite to faint yellow powderWhite to faint yellow powderWhite to faint yellow powderWhite to faint yellow powderWhite to faint yellow powder
-
PurityHPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%
-
Storage Condition-20℃-20℃-20℃-20℃-20℃-20℃-20℃-20℃
-
Moisture ContentK.F.≤0.20% w/wK.F.≤0.20% w/wK.F.≤0.20% w/wK.F.≤0.20% w/wK.F.≤0.20% w/wK.F.≤0.20% w/wK.F.≤0.20% w/wK.F.≤0.20% w/w
Why choose Hongene?
Trusted Partner in Nucleic Acid
Integrated Supply & Commercial Scale
With 26+ years of expertise, we control a secure supply chain for RNA raw materials and provide reliable GMP-grade oligo synthesis from research to commercial kilogram-scale production.
Proprietary Technology & IP
Our proprietary Chemoenzymatic Ligation Platform combines chemical andenzymatic methods, enabling high-putity, cost-effective, and large-scale production of RNA-based therapeutics.
Rigorous Quality
We implement multiple stringent QC steps, maintain ISO certifications, and ensure >99% batch-to-batch consistency, reducing scale-up and PPQ risks.
Manufacturing Scalability
Hongene operates 1.67 million sq. ft Oligonucleotide Manufacturing Facility, with advanced equipments including multiple OligoPilot™ and OligoProcess™ synthesizers (10-1800 mmol). 48 flexible production lines enable one-stop seamless scaling-up of API production from gram-level to tons and acheive high purity of 98%, meeting NMPA, FDA, and EMA standards.
Global Business Network
Our products and services reach over 40 countries and regions, supporting around 3,000 clients worldwide.