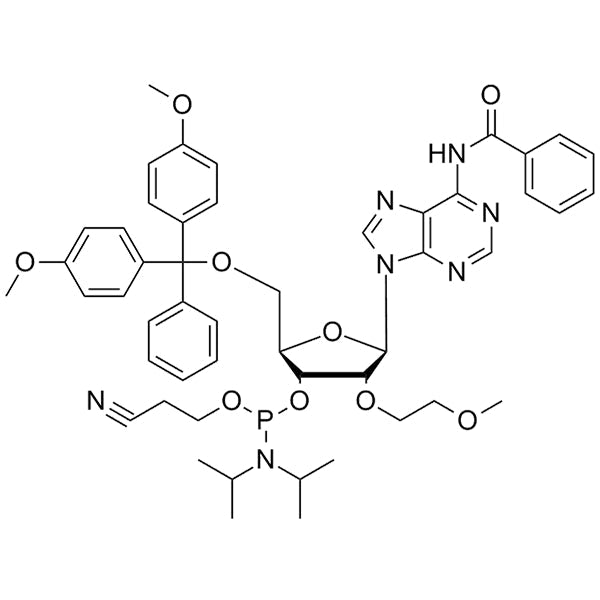
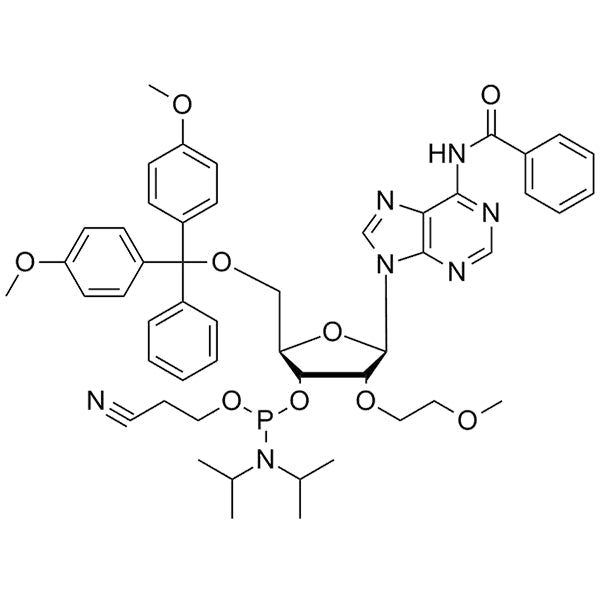
DMT-2'-O-MOE-A(Bz)-CE-Phosphoramidite
DMT-2'-O-MOE-A(Bz)-CE-Phosphoramidite - N (Normal) / 0.25g / 30mL screwed bottle-28 is backordered and will ship as soon as it is back in stock.
Couldn't load pickup availability
Shipping notes
Shipping notes
Products in shop.hongene.com currently can only ship to the United States and Canada. For other regions, please visit contact us page to leave us a message or contact our regional sales.
Processing Time: within 2 business days, if available.
Delivery Time:- US: 3-8 business days (FedEx Standard).
- Canada: 5-10 business days (excludes customs delays).
Note: Delays due to force majeure like customs, natural disasters, or strikes are beyond our liability.
Excerpt from Shipping Policy
Related Products
Details
Details
Application
The incorporation of 2′O-Methyl RNA nucleoside, including DMT-2′O-MOE-A(Bz)-C-phosphoramidite, in nucleic acid probes with RNA or DNA is advantageous for in-vivo or in-vitro applications, as it allows for the delivery of nuclease-resistant materials.
Features and Benefits
The product's key features are listed below:
- High yield of crude oligonucleotides
- Compatible with DNA synthesis
- Can be used in conjunction with DNA or RNA phosphoramidites in the same synthesis to produce mixed oligonucleotides
- Recommended deprotection conditions are 8 hours at 55 °C using concentrated ammonia solution, or with AMA (concentrated ammonia/ 40% aqueous methylamine I/I, v/v) for 10 minutes at 65 °C
- Purification and other downstream processing of fully modified 2′OMethyl RNA oligonucleotides are simpler than in the case of RNA, as no special precautions are required to provide protection against nucleolytic degradation
- Synthesis of 2′O-Methyl RNA oligonucleotides is similar to standard DNA synthesis but requires an elongated coupling time (recommended is 6 minutes compared to 90 seconds for DNA monomers)
- 2′O-Methyl RNA phosphoramidites are also available with fast deprotection chemistry
Other Notes
The distinctive characteristics of 2′O-methyl RNA have led to its extensive utilization in various fields, including:
- Diagnostic probes
- Aptamer and ribozyme development
- Mixed 2′O-Methyl-RNA/DNA antisense molecules
Specifications
Specifications
-
Catalog No.PR1-004PR1-004PR1-004PR1-004PR1-004PR1-004PR1-004PR1-004
-
CAS No.251647-53-7251647-53-7251647-53-7251647-53-7251647-53-7251647-53-7251647-53-7251647-53-7
-
SMILESCC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(C4=CC=CC=C4)=O)=NC=N3)O[C@@H]1COC(C5=CC=C(OC)C=C5)(C6=CC=CC=C6)C7=CC=C(OC)C=C7CC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(C4=CC=CC=C4)=O)=NC=N3)O[C@@H]1COC(C5=CC=C(OC)C=C5)(C6=CC=CC=C6)C7=CC=C(OC)C=C7CC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(C4=CC=CC=C4)=O)=NC=N3)O[C@@H]1COC(C5=CC=C(OC)C=C5)(C6=CC=CC=C6)C7=CC=C(OC)C=C7CC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(C4=CC=CC=C4)=O)=NC=N3)O[C@@H]1COC(C5=CC=C(OC)C=C5)(C6=CC=CC=C6)C7=CC=C(OC)C=C7CC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(C4=CC=CC=C4)=O)=NC=N3)O[C@@H]1COC(C5=CC=C(OC)C=C5)(C6=CC=CC=C6)C7=CC=C(OC)C=C7CC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(C4=CC=CC=C4)=O)=NC=N3)O[C@@H]1COC(C5=CC=C(OC)C=C5)(C6=CC=CC=C6)C7=CC=C(OC)C=C7CC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(C4=CC=CC=C4)=O)=NC=N3)O[C@@H]1COC(C5=CC=C(OC)C=C5)(C6=CC=CC=C6)C7=CC=C(OC)C=C7CC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(C4=CC=CC=C4)=O)=NC=N3)O[C@@H]1COC(C5=CC=C(OC)C=C5)(C6=CC=CC=C6)C7=CC=C(OC)C=C7
-
Molecular FormulaC50H58N7O9PC50H58N7O9PC50H58N7O9PC50H58N7O9PC50H58N7O9PC50H58N7O9PC50H58N7O9PC50H58N7O9P
-
Molecular Weight932.03932.03932.03932.03932.03932.03932.03932.03
-
AppearanceWhite to off-white powderWhite to off-white powderWhite to off-white powderWhite to off-white powderWhite to off-white powderWhite to off-white powderWhite to off-white powderWhite to off-white powder
-
PurityHPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%
-
Storage Condition-20℃-20℃-20℃-20℃-20℃-20℃-20℃-20℃
Documentation
Documentation
Application
The incorporation of 2′O-Methyl RNA nucleoside, including DMT-2′O-MOE-A(Bz)-C-phosphoramidite, in nucleic acid probes with RNA or DNA is advantageous for in-vivo or in-vitro applications, as it allows for the delivery of nuclease-resistant materials.
Features and Benefits
The product's key features are listed below:
- High yield of crude oligonucleotides
- Compatible with DNA synthesis
- Can be used in conjunction with DNA or RNA phosphoramidites in the same synthesis to produce mixed oligonucleotides
- Recommended deprotection conditions are 8 hours at 55 °C using concentrated ammonia solution, or with AMA (concentrated ammonia/ 40% aqueous methylamine I/I, v/v) for 10 minutes at 65 °C
- Purification and other downstream processing of fully modified 2′OMethyl RNA oligonucleotides are simpler than in the case of RNA, as no special precautions are required to provide protection against nucleolytic degradation
- Synthesis of 2′O-Methyl RNA oligonucleotides is similar to standard DNA synthesis but requires an elongated coupling time (recommended is 6 minutes compared to 90 seconds for DNA monomers)
- 2′O-Methyl RNA phosphoramidites are also available with fast deprotection chemistry
Other Notes
The distinctive characteristics of 2′O-methyl RNA have led to its extensive utilization in various fields, including:
- Diagnostic probes
- Aptamer and ribozyme development
- Mixed 2′O-Methyl-RNA/DNA antisense molecules
-
Catalog No.PR1-004PR1-004PR1-004PR1-004PR1-004PR1-004PR1-004PR1-004
-
CAS No.251647-53-7251647-53-7251647-53-7251647-53-7251647-53-7251647-53-7251647-53-7251647-53-7
-
SMILESCC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(C4=CC=CC=C4)=O)=NC=N3)O[C@@H]1COC(C5=CC=C(OC)C=C5)(C6=CC=CC=C6)C7=CC=C(OC)C=C7CC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(C4=CC=CC=C4)=O)=NC=N3)O[C@@H]1COC(C5=CC=C(OC)C=C5)(C6=CC=CC=C6)C7=CC=C(OC)C=C7CC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(C4=CC=CC=C4)=O)=NC=N3)O[C@@H]1COC(C5=CC=C(OC)C=C5)(C6=CC=CC=C6)C7=CC=C(OC)C=C7CC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(C4=CC=CC=C4)=O)=NC=N3)O[C@@H]1COC(C5=CC=C(OC)C=C5)(C6=CC=CC=C6)C7=CC=C(OC)C=C7CC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(C4=CC=CC=C4)=O)=NC=N3)O[C@@H]1COC(C5=CC=C(OC)C=C5)(C6=CC=CC=C6)C7=CC=C(OC)C=C7CC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(C4=CC=CC=C4)=O)=NC=N3)O[C@@H]1COC(C5=CC=C(OC)C=C5)(C6=CC=CC=C6)C7=CC=C(OC)C=C7CC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(C4=CC=CC=C4)=O)=NC=N3)O[C@@H]1COC(C5=CC=C(OC)C=C5)(C6=CC=CC=C6)C7=CC=C(OC)C=C7CC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(C4=CC=CC=C4)=O)=NC=N3)O[C@@H]1COC(C5=CC=C(OC)C=C5)(C6=CC=CC=C6)C7=CC=C(OC)C=C7
-
Molecular FormulaC50H58N7O9PC50H58N7O9PC50H58N7O9PC50H58N7O9PC50H58N7O9PC50H58N7O9PC50H58N7O9PC50H58N7O9P
-
Molecular Weight932.03932.03932.03932.03932.03932.03932.03932.03
-
AppearanceWhite to off-white powderWhite to off-white powderWhite to off-white powderWhite to off-white powderWhite to off-white powderWhite to off-white powderWhite to off-white powderWhite to off-white powder
-
PurityHPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%
-
Storage Condition-20℃-20℃-20℃-20℃-20℃-20℃-20℃-20℃
Why choose Hongene?
Trusted Partner in Nucleic Acid
Integrated Supply & Commercial Scale
With 26+ years of expertise, we control a secure supply chain for RNA raw materials and provide reliable GMP-grade oligo synthesis from research to commercial kilogram-scale production.
Proprietary Technology & IP
Our proprietary Chemoenzymatic Ligation Platform combines chemical andenzymatic methods, enabling high-putity, cost-effective, and large-scale production of RNA-based therapeutics.
Rigorous Quality
We implement multiple stringent QC steps, maintain ISO certifications, and ensure >99% batch-to-batch consistency, reducing scale-up and PPQ risks.
Manufacturing Scalability
Hongene operates 1.67 million sq. ft Oligonucleotide Manufacturing Facility, with advanced equipments including multiple OligoPilot™ and OligoProcess™ synthesizers (10-1800 mmol). 48 flexible production lines enable one-stop seamless scaling-up of API production from gram-level to tons and acheive high purity of 98%, meeting NMPA, FDA, and EMA standards.
Global Business Network
Our products and services reach over 40 countries and regions, supporting around 3,000 clients worldwide.