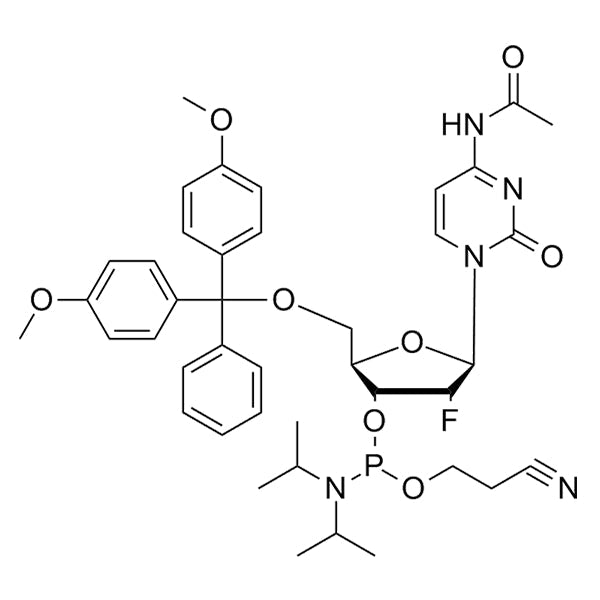
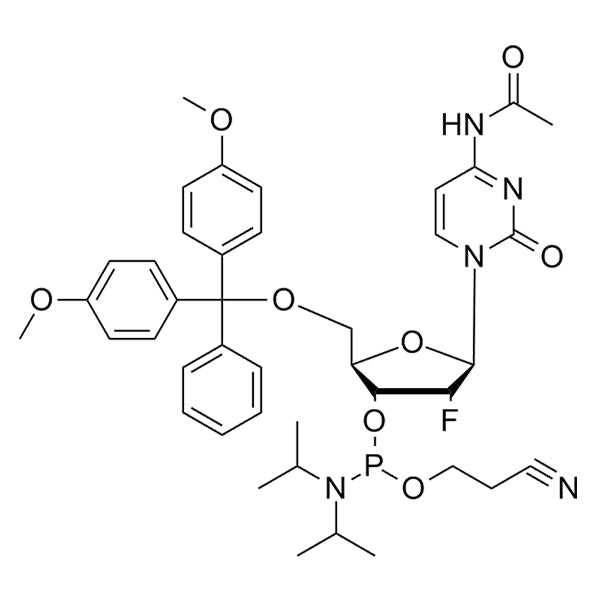
DMT-2'-F-dC(Ac)-CE Phosphoramidite
DMT-2'-F-dC(Ac)-CE Phosphoramidite - N (Normal) / 0.25g / 30mL screwed bottle-28 is backordered and will ship as soon as it is back in stock.
Couldn't load pickup availability
Shipping notes
Shipping notes
Related Products
Details
Details
DMT-2’-F-dC(Ac)-CE-Phosphoramidite is a 2'-fluoro (2'-F) phosphoramidite monomer used for solid-phase oligonucleotide synthesis.
It contains a 2'-F modification on the sugar of deoxycytidine (dC) with acetyl (Ac) base protection, a 5’-dimethoxytrityl (DMT) group for 5'-hydroxyl protection, and a cyanoethyl (CE) protecting group on phosphite.
Synthesis of RNA oligonucleotides using 2'-F phosphoramidite increases the nuclease resistance and binding affinity of duplexes formed with complementary sequences.
Applications
Therapeutic oligonucleotides
Commonly used in chemically modified small interfering RNA (siRNA) to enhance stability, potency, and in-vivo performance.[1][2]
Probing and diagnostics
For constructing nuclease-resistant and high-affinity probes (e.g, aptamers) to study biomolecule interactions,[3][4] which can be related to diseases and cancers.[5][6]
Features and Benefits
Other Notes
- Diluent: Anhydrous Acetonitrile.
- Storage: Store in a dry and inert atmosphere at -20 °C.
- Coupling: 6 minute coupling time recommended.
Reference
Specifications
Specifications
-
Catalog No.PD3-001PD3-001PD3-001PD3-001PD3-001PD3-001PD3-001PD3-001
-
CAS No.159414-99-0159414-99-0159414-99-0159414-99-0159414-99-0159414-99-0159414-99-0159414-99-0
-
SMILESF[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=OF[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=OF[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=OF[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=OF[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=OF[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=OF[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=OF[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=O
-
Molecular FormulaC41H49FN5O8PC41H49FN5O8PC41H49FN5O8PC41H49FN5O8PC41H49FN5O8PC41H49FN5O8PC41H49FN5O8PC41H49FN5O8P
-
Molecular Weight789.83789.83789.83789.83789.83789.83789.83789.83
-
AppearanceWhite powderWhite powderWhite powderWhite powderWhite powderWhite powderWhite powderWhite powder
-
PurityHPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%
-
Storage Condition-20℃-20℃-20℃-20℃-20℃-20℃-20℃-20℃
-
Moisture ContentK.F.≤0.20% w/wK.F.≤0.20% w/wK.F.≤0.20% w/wK.F.≤0.20% w/wK.F.≤0.20% w/wK.F.≤0.20% w/wK.F.≤0.20% w/wK.F.≤0.20% w/w
Documentation
Documentation
DMT-2’-F-dC(Ac)-CE-Phosphoramidite is a 2'-fluoro (2'-F) phosphoramidite monomer used for solid-phase oligonucleotide synthesis.
It contains a 2'-F modification on the sugar of deoxycytidine (dC) with acetyl (Ac) base protection, a 5’-dimethoxytrityl (DMT) group for 5'-hydroxyl protection, and a cyanoethyl (CE) protecting group on phosphite.
Synthesis of RNA oligonucleotides using 2'-F phosphoramidite increases the nuclease resistance and binding affinity of duplexes formed with complementary sequences.
Applications
Therapeutic oligonucleotides
Commonly used in chemically modified small interfering RNA (siRNA) to enhance stability, potency, and in-vivo performance.[1][2]
Probing and diagnostics
For constructing nuclease-resistant and high-affinity probes (e.g, aptamers) to study biomolecule interactions,[3][4] which can be related to diseases and cancers.[5][6]
Features and Benefits
Other Notes
- Diluent: Anhydrous Acetonitrile.
- Storage: Store in a dry and inert atmosphere at -20 °C.
- Coupling: 6 minute coupling time recommended.
Reference
-
Catalog No.PD3-001PD3-001PD3-001PD3-001PD3-001PD3-001PD3-001PD3-001
-
CAS No.159414-99-0159414-99-0159414-99-0159414-99-0159414-99-0159414-99-0159414-99-0159414-99-0
-
SMILESF[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=OF[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=OF[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=OF[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=OF[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=OF[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=OF[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=OF[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(C2=CC=C(OC)C=C2)(C3=CC=CC=C3)C4=CC=C(OC)C=C4)O[C@H]1N5C(N=C(NC(C)=O)C=C5)=O
-
Molecular FormulaC41H49FN5O8PC41H49FN5O8PC41H49FN5O8PC41H49FN5O8PC41H49FN5O8PC41H49FN5O8PC41H49FN5O8PC41H49FN5O8P
-
Molecular Weight789.83789.83789.83789.83789.83789.83789.83789.83
-
AppearanceWhite powderWhite powderWhite powderWhite powderWhite powderWhite powderWhite powderWhite powder
-
PurityHPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%
-
Storage Condition-20℃-20℃-20℃-20℃-20℃-20℃-20℃-20℃
-
Moisture ContentK.F.≤0.20% w/wK.F.≤0.20% w/wK.F.≤0.20% w/wK.F.≤0.20% w/wK.F.≤0.20% w/wK.F.≤0.20% w/wK.F.≤0.20% w/wK.F.≤0.20% w/w
Why choose Hongene?
Trusted Partner in Nucleic Acid
Integrated Supply & Commercial Scale
With 26+ years of expertise, we control a secure supply chain for RNA raw materials and provide reliable GMP-grade oligo synthesis from research to commercial kilogram-scale production.
Proprietary Technology & IP
Our proprietary Chemoenzymatic Ligation Platform combines chemical andenzymatic methods, enabling high-putity, cost-effective, and large-scale production of RNA-based therapeutics.
Rigorous Quality
We implement multiple stringent QC steps, maintain ISO certifications, and ensure >99% batch-to-batch consistency, reducing scale-up and PPQ risks.
Manufacturing Scalability
Hongene operates 1.67 million sq. ft Oligonucleotide Manufacturing Facility, with advanced equipments including multiple OligoPilot™ and OligoProcess™ synthesizers (10-1800 mmol). 48 flexible production lines enable one-stop seamless scaling-up of API production from gram-level to tons and acheive high purity of 98%, meeting NMPA, FDA, and EMA standards.
Global Business Network
Our products and services reach over 40 countries and regions, supporting around 3,000 clients worldwide.