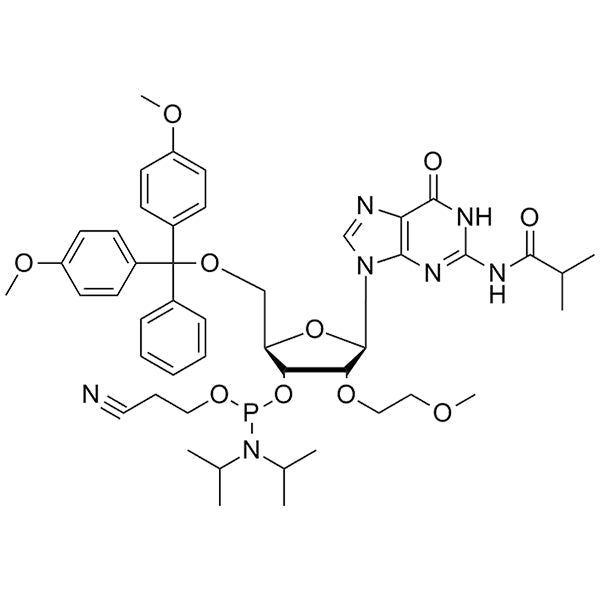
DMT-2'-O-MOE-G(iBu)-CE-Phosphoramidite
DMT-2'-O-MOE-G(iBu)-CE-Phosphoramidite - N (Normal) / 0.25g / 30mL screwed bottle-28 is backordered and will ship as soon as it is back in stock.
Couldn't load pickup availability
Shipping notes
Shipping notes
Related Products
Details
Details
DMT-2'-O-MOE-G(iBu)-CE-Phosphoramidite is a 2'-MOE phosphoramidite monomer used for solid-phase oligonucleotide synthesis. It contains a 5'-dimethoxytrityl (DMT) protecting group for 5'-hydroxyl protection, a isobutyryl (iBu) base-protecting group on guanine, a cyanoethyl (CE) protecting group on phosphite, and a 2'-O-methoxyethyl (MOE) modification.
2'-O-MOE provide enhanced nuclease resistance, duplex stability, and low cytotoxicity compared with unmodified RNA.
Structurally, the 2'-hydroxyl group of ribose is replaced by a –O–CH₂–CH₂–O–CH₃ (methoxyethyl) group, which locks the sugar into an RNA-like C3'-endo conformation and stabilizes hybrid formation with complementary RNA strands.
Applications
RNase H-dependent antisense oligonucleotides (ASO)
Often as 2'-O-MOE/DNA chimeras that activate RNase H-mediated cleavage of target RNA.[1]
Steric-blocking ASO
Physically obstructing access to specific RNA molecules, rather than causing their degradation, e.g., splice-switching ASO.[1]
Enzyme or structural RNA inhibition
Inhibit specific RNAs or enzymes (e.g., telomerase) through binding interference.[2]
Features and Benefits
Other Notes
- Storage: Store in a dry, inert atmosphere at -20 °C.
- Coupling: 6 minute coupling time recommended
- Recommended deprotection: 8 h at 55 °C in concentrated ammonia, or 10 min at 65 °C in AMA (ammonia/methylamine, 1:1 v/v)
- Compatibility: Can be used alongside modified phosphoramidites (e.g., 2′-OMe, 2′-MOE, Locked-NA) to synthesize chimeric oligonucleotides.
Reference
[1] Hill, Alyssa C., and Jonathan Hall. "The MOE modification of RNA: Origins and widescale impact on the oligonucleotide therapeutics field." Helvetica Chimica Acta 106, no. 3 (2023): e202200169.
[2] Elayadi, Anissa N., Andrea Demieville, Edward V. Wancewicz, Brett P. Monia, and David R. Corey. "Inhibition of telomerase by 2′-O-(2-methoxyethyl) RNA oligomers: effect of length, phosphorothioate substitution and time inside cells." Nucleic Acids Research 29, no. 8 (2001): 1683-1689.
Specifications
Specifications
-
Catalog No.PR2-006PR2-006PR2-006PR2-006PR2-006PR2-006PR2-006PR2-006
-
CAS No.251647-55-9251647-55-9251647-55-9251647-55-9251647-55-9251647-55-9251647-55-9251647-55-9
-
SMILESCC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(NC(C(C)C)=O)=N3)=O)O[C@@H]1COC(C4=CC=C(OC)C=C4)(C5=CC=CC=C5)C6=CC=C(OC)C=C6CC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(NC(C(C)C)=O)=N3)=O)O[C@@H]1COC(C4=CC=C(OC)C=C4)(C5=CC=CC=C5)C6=CC=C(OC)C=C6CC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(NC(C(C)C)=O)=N3)=O)O[C@@H]1COC(C4=CC=C(OC)C=C4)(C5=CC=CC=C5)C6=CC=C(OC)C=C6CC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(NC(C(C)C)=O)=N3)=O)O[C@@H]1COC(C4=CC=C(OC)C=C4)(C5=CC=CC=C5)C6=CC=C(OC)C=C6CC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(NC(C(C)C)=O)=N3)=O)O[C@@H]1COC(C4=CC=C(OC)C=C4)(C5=CC=CC=C5)C6=CC=C(OC)C=C6CC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(NC(C(C)C)=O)=N3)=O)O[C@@H]1COC(C4=CC=C(OC)C=C4)(C5=CC=CC=C5)C6=CC=C(OC)C=C6CC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(NC(C(C)C)=O)=N3)=O)O[C@@H]1COC(C4=CC=C(OC)C=C4)(C5=CC=CC=C5)C6=CC=C(OC)C=C6CC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(NC(C(C)C)=O)=N3)=O)O[C@@H]1COC(C4=CC=C(OC)C=C4)(C5=CC=CC=C5)C6=CC=C(OC)C=C6
-
Molecular FormulaC47H60N7O10PC47H60N7O10PC47H60N7O10PC47H60N7O10PC47H60N7O10PC47H60N7O10PC47H60N7O10PC47H60N7O10P
-
Molecular Weight914.01914.01914.01914.01914.01914.01914.01914.01
-
AppearanceWhite to off-white powderWhite to off-white powderWhite to off-white powderWhite to off-white powderWhite to off-white powderWhite to off-white powderWhite to off-white powderWhite to off-white powder
-
PurityHPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%
-
Storage Condition-20℃-20℃-20℃-20℃-20℃-20℃-20℃-20℃
-
Moisture ContentK.F.≤0.30% w/wK.F.≤0.30% w/wK.F.≤0.30% w/wK.F.≤0.30% w/wK.F.≤0.30% w/wK.F.≤0.30% w/wK.F.≤0.30% w/wK.F.≤0.30% w/w
Documentation
Documentation
DMT-2'-O-MOE-G(iBu)-CE-Phosphoramidite is a 2'-MOE phosphoramidite monomer used for solid-phase oligonucleotide synthesis. It contains a 5'-dimethoxytrityl (DMT) protecting group for 5'-hydroxyl protection, a isobutyryl (iBu) base-protecting group on guanine, a cyanoethyl (CE) protecting group on phosphite, and a 2'-O-methoxyethyl (MOE) modification.
2'-O-MOE provide enhanced nuclease resistance, duplex stability, and low cytotoxicity compared with unmodified RNA.
Structurally, the 2'-hydroxyl group of ribose is replaced by a –O–CH₂–CH₂–O–CH₃ (methoxyethyl) group, which locks the sugar into an RNA-like C3'-endo conformation and stabilizes hybrid formation with complementary RNA strands.
Applications
RNase H-dependent antisense oligonucleotides (ASO)
Often as 2'-O-MOE/DNA chimeras that activate RNase H-mediated cleavage of target RNA.[1]
Steric-blocking ASO
Physically obstructing access to specific RNA molecules, rather than causing their degradation, e.g., splice-switching ASO.[1]
Enzyme or structural RNA inhibition
Inhibit specific RNAs or enzymes (e.g., telomerase) through binding interference.[2]
Features and Benefits
Other Notes
- Storage: Store in a dry, inert atmosphere at -20 °C.
- Coupling: 6 minute coupling time recommended
- Recommended deprotection: 8 h at 55 °C in concentrated ammonia, or 10 min at 65 °C in AMA (ammonia/methylamine, 1:1 v/v)
- Compatibility: Can be used alongside modified phosphoramidites (e.g., 2′-OMe, 2′-MOE, Locked-NA) to synthesize chimeric oligonucleotides.
Reference
[1] Hill, Alyssa C., and Jonathan Hall. "The MOE modification of RNA: Origins and widescale impact on the oligonucleotide therapeutics field." Helvetica Chimica Acta 106, no. 3 (2023): e202200169.
[2] Elayadi, Anissa N., Andrea Demieville, Edward V. Wancewicz, Brett P. Monia, and David R. Corey. "Inhibition of telomerase by 2′-O-(2-methoxyethyl) RNA oligomers: effect of length, phosphorothioate substitution and time inside cells." Nucleic Acids Research 29, no. 8 (2001): 1683-1689.
-
Catalog No.PR2-006PR2-006PR2-006PR2-006PR2-006PR2-006PR2-006PR2-006
-
CAS No.251647-55-9251647-55-9251647-55-9251647-55-9251647-55-9251647-55-9251647-55-9251647-55-9
-
SMILESCC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(NC(C(C)C)=O)=N3)=O)O[C@@H]1COC(C4=CC=C(OC)C=C4)(C5=CC=CC=C5)C6=CC=C(OC)C=C6CC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(NC(C(C)C)=O)=N3)=O)O[C@@H]1COC(C4=CC=C(OC)C=C4)(C5=CC=CC=C5)C6=CC=C(OC)C=C6CC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(NC(C(C)C)=O)=N3)=O)O[C@@H]1COC(C4=CC=C(OC)C=C4)(C5=CC=CC=C5)C6=CC=C(OC)C=C6CC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(NC(C(C)C)=O)=N3)=O)O[C@@H]1COC(C4=CC=C(OC)C=C4)(C5=CC=CC=C5)C6=CC=C(OC)C=C6CC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(NC(C(C)C)=O)=N3)=O)O[C@@H]1COC(C4=CC=C(OC)C=C4)(C5=CC=CC=C5)C6=CC=C(OC)C=C6CC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(NC(C(C)C)=O)=N3)=O)O[C@@H]1COC(C4=CC=C(OC)C=C4)(C5=CC=CC=C5)C6=CC=C(OC)C=C6CC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(NC(C(C)C)=O)=N3)=O)O[C@@H]1COC(C4=CC=C(OC)C=C4)(C5=CC=CC=C5)C6=CC=C(OC)C=C6CC(C)N(C(C)C)P(OCCC#N)O[C@H]1[C@@H](OCCOC)[C@H](N(C=N2)C3=C2C(NC(NC(C(C)C)=O)=N3)=O)O[C@@H]1COC(C4=CC=C(OC)C=C4)(C5=CC=CC=C5)C6=CC=C(OC)C=C6
-
Molecular FormulaC47H60N7O10PC47H60N7O10PC47H60N7O10PC47H60N7O10PC47H60N7O10PC47H60N7O10PC47H60N7O10PC47H60N7O10P
-
Molecular Weight914.01914.01914.01914.01914.01914.01914.01914.01
-
AppearanceWhite to off-white powderWhite to off-white powderWhite to off-white powderWhite to off-white powderWhite to off-white powderWhite to off-white powderWhite to off-white powderWhite to off-white powder
-
PurityHPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%HPLC≥98.0%
-
Storage Condition-20℃-20℃-20℃-20℃-20℃-20℃-20℃-20℃
-
Moisture ContentK.F.≤0.30% w/wK.F.≤0.30% w/wK.F.≤0.30% w/wK.F.≤0.30% w/wK.F.≤0.30% w/wK.F.≤0.30% w/wK.F.≤0.30% w/wK.F.≤0.30% w/w
Why choose Hongene?
Trusted Partner in Nucleic Acid
Integrated Supply & Commercial Scale
With 26+ years of expertise, we control a secure supply chain for RNA raw materials and provide reliable GMP-grade oligo synthesis from research to commercial kilogram-scale production.
Proprietary Technology & IP
Our proprietary Chemoenzymatic Ligation Platform combines chemical andenzymatic methods, enabling high-putity, cost-effective, and large-scale production of RNA-based therapeutics.
Rigorous Quality
We implement multiple stringent QC steps, maintain ISO certifications, and ensure >99% batch-to-batch consistency, reducing scale-up and PPQ risks.
Manufacturing Scalability
Hongene operates 1.67 million sq. ft Oligonucleotide Manufacturing Facility, with advanced equipments including multiple OligoPilot™ and OligoProcess™ synthesizers (10-1800 mmol). 48 flexible production lines enable one-stop seamless scaling-up of API production from gram-level to tons and acheive high purity of 98%, meeting NMPA, FDA, and EMA standards.
Global Business Network
Our products and services reach over 40 countries and regions, supporting around 3,000 clients worldwide.